If you’re preparing for the 2024 NECO GCE Chemistry (Alternative to Practical) exam, this article provides all the information you need. You’ll find the NECO GCE Chemistry Practical questions and answers for 2024, along with tips and exam details to help you succeed.
We offer valuable insights for the NECO GCE Chemistry (Alternative to Practical) exam, making it easy to understand the format and key areas to focus on. Whether you’re looking for the 2024 NECO GCE Chemistry (Alternative to Practical) questions and answers for the November/December exams or want to learn more about the exam format, this guide is designed to help you prepare effectively.
To access free Questions and answers, simply subscribe to our YouTube channel by clicking the “Subscribe Now” button ✅ below 👇👇👇.
Also Watch chemistry videos below:
Download NECO GCE Chemistry (Alternative to Practical) Questions and Answers for 2024
You can also download the NECO GCE Chemistry Practical answers for 2024 as a PDF for offline study. We’ve gathered helpful resources to assist you in understanding the questions and improving your preparation. Use this guide to enhance your study and perform at your best in the exam.

Important Dates:
The Chemistry Paper I (Alternative to Practical) exam is scheduled for Monday, 18th November 2024, from 2:00 PM – 4:00 PM. The exact timing will be confirmed by the National Examinations Council (NECO).
NOV/DEC 2024 NECO GCE Chemistry Paper I (Alternative to Practical) Questions and Answers
The resources listed below for Chemistry Practical will help you understand what to expect in the final exams. These materials are essential for students aiming to succeed.
Iyierioba.com has carefully analyzed student performance in past exams, providing useful insights and tips for effective preparation.
By reviewing these resources, you can identify common challenges and successful strategies, giving you a better understanding of what to focus on. This will help improve your comprehension and increase your chances of scoring highly in the exams. Use these tools to guide your study and approach the exam with confidence.
NECO GCE Chemistry Alternative to Practical | 28th Nov 2024



=====================================


====================================
====================================
[ANSWERS LOADING =========================]
CLICK HERE TO JOIN OUR TELEGRAM CHANNEL
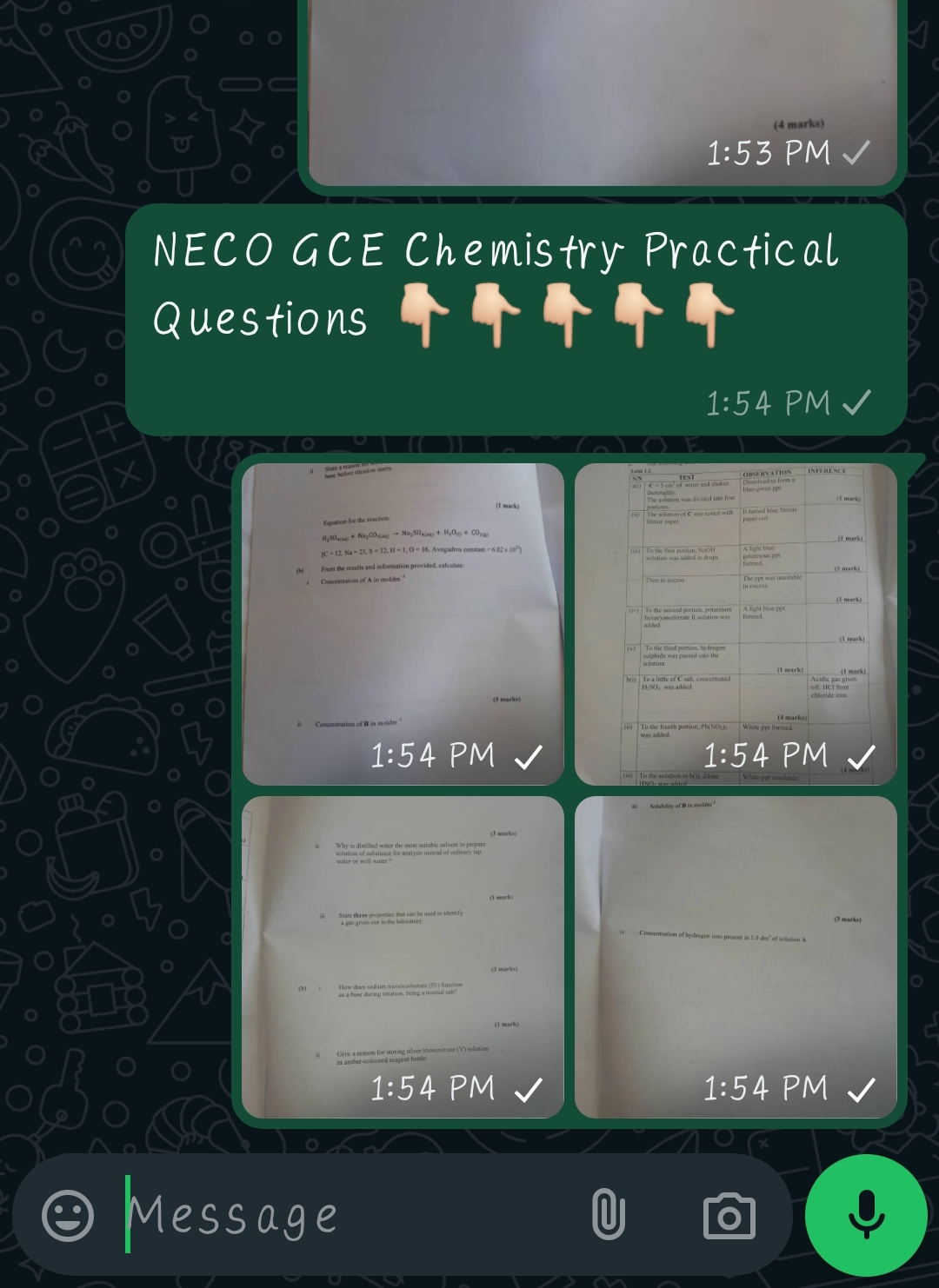
NECO GCE chemistry practical Instructions
Each candidate should be supplied with the following: A, B, C, and D.- Where ‘n’ is the candidate’s serial number.
(a) 150 cm3 of tetraoxosulphate(VI) acid solution in a bottle labelled “A.. The acid solution which should be the same for all candidates will contain 3.8 cm3 of concentrated tetraoxosulphate(VI) acid per dm3 of solution.
(b) 150 cm3 of sodium trioxocarbonate(IV) solution in a bottle labelled “B,”. The solution which should be the same for all candidates will contain 6.00 g of sodium trioxocarbonate(TV) per dm’ of solution.
(c) One spatulaful of zinc chloride salt in a specimen bottle labelled C
(d) Solution of powdered milk in a specimen bottle labelled D..
5. In all cases, more materials may be given if required.
6. The actual concentrations of A and B must be stated on the Subject Teacher’s Report Form, while the candidates should make use of the concentrations stated in the question paper.
7. Please, do not substitute any substance or solution for those specified in these instructions
ARRANGEMENT AND SERIAL NUMBERS OF CANDIDATES
8. The place for work by candidates in the laboratory should be numbered serially and the candidates allotted these places strictly in order of their examination numbers.
9. The number of every specimen supplied to each candidate should tally with his/her serial number. If a candidate is absent, his/her serial number must not be allotted to another candidate.
10. If candidates are divided into sets, the serial number should be continued through the sets to ensure that no serial number is repeated.
11. Ensure that the candidates record their serial numbers as well as their examination numbers on the front of their answer booklets at the beginning of the examination.
TEACHER’S REPORT
12. Ensure that a completed Teacher’s Report Form is enclosed in each envelop of scripts. This report form is provided separately.
13. Carry out the titration required of the candidates in question I and record the titre values which should be at least one decimal place in the spaces provided for these in the Teacher’s Report Form. Verify the titre values before recording them.
RECOMMENDED POSTS:
- NECO GCE Igbo, Yoruba & Hausa (Objective & Essay) Answers – 22nd Nov 2024
- 2024 NECO GCE History (Objective/Essay) Answers | 26th Nov
- 2024 NECO GCE Further Mathematics (Objective/essay) Questions and Answers
- NECO GCE Arabic (Oral) Questions and Answers – 21st Nov 2024
- NECO French (Oral) Questions and Answer | 21st Nov 2024
- NECO GCE 2024: Subjects, How to Apply, and Registration Guide
DISCLAIMER: This article is provided for educational purposes only. We do not promote or encourage exam malpractice.

