2024 NECO CHEMISTRY PRACTICAL QUESTIONS AND ANSWERS:
NECO 2024 Update
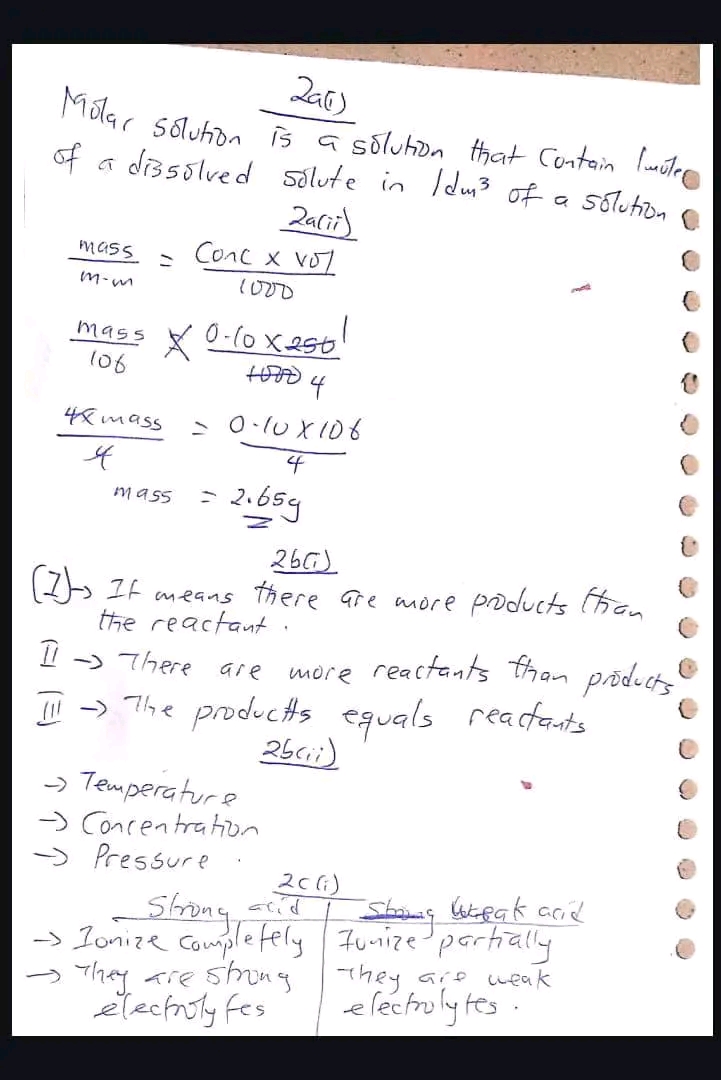
Remember
You can still subscribe for NECO.Important
The price will go up next week!Subscribe Now
Pay now and get:
Added to NECO VIP Group
Answers sent to you from midnight
Subscribe now, don’t delay!
NOTE: NECO June/July 2024 Free Chemistry Practical (Specimen) Question and Answer Room: Pay just N1,500! Click on the link below to WhatsApp us and get the answers at 12 midnight. Prepare effectively and ensure your success in the exam with our comprehensive resources.
Click here to join WhatsApp group
2024 NECO CHEMISTRY OBJ/ ESSAY QUESTIONS AND ANSWERS:

Answers Loading…………..
2024 NECO CHEMISTRY ESSAY
NUMBER 1
(1a)
(i) Hydrogen gas is colorless and odorless.
(ii) Hydrogen is highly flammable, which means it can easily catch fire when exposed to a flame or spark.
(iii) Hydrogen gas has low density, making it lighter than air.
(1b)
(I) Group 17 (halogens)
(II) lodine
(III) lodine
(1ci)
I. Calcium (Ca²⁺)
II. Magnesium (Mg²⁺)
(1cii)
I. lon exchange method
II. Lime-soda method
(1di)
(I) Methyl orange is used in the titration of a strong acid with a strong base.
(II) Phenolphthalein is used in the titration of a strong base with a strong acid.
(1dii)
(I) Hydrated salt: Copper(II) sulfate pentahydrate (CuSO₄.5H₂O)
(II) Acidic salt: Ammonium chloride (NH₄Cl)
(III) Basic salt: Sodium carbonate (Na₂CO₃)
(1ei)
2-methylbutane –> pentane –> 2,2-dimethylpropane
(1eii)
(I) 1-bromo-3-methylbutane
(II) 2-methylpentane
(1eiii)
Benzene is used as a starting material in the production of various important chemicals such as ethylbenzene, cumene, cyclohexane, and nitrobenzene.
(1eiv)
Saponification is a process in which ester molecules are hydrolyzed in the presence of a strong base (such as sodium hydroxide or potassium hydroxide) to form alcohol and the salt of the carboxylic acid.
(1f)
Given, Vapour density = 77
molecular mass of XCl₄ = Molecular mass = Vapour density x 2 = 77 x 2 = 154
Molecular mass of XCl₄ = X + 4(Atomic mass of Cl)
154 = X + 4(35.5)
X = 154 – 142
X = 12
:. The relative atomic mass of X is 12.
====================
CLICK HERE TO GET FULL ANSWERS
2024 NECO CHEMISTRY PRACTICAL QUESTIONS AND ANSWERS:


2024 NECO CHEMISTRY PRACTICAL ANSWERS:
NUMBER 1
Solving/Typing…
====================
NUMBER 2
Solving/Typing…
===================
NUMBER 3
(3ai)
I- to avoid the drop of the acid into the burette which will alter the reading
II- to avoid change in concentration and contamination
III- to avoid bubbles in the pipette
(3aii)
This can be done through crystallization. The solution is dissolved, heated to a high temperature and then allowed to cool. During cooling,the sodium chloride crystallized out.
(3bi)
(i)evaporating dish
(ii)Bunsen burner
(3bii)
Solubility
====================
Answers Loading………………………………………
👇👇👇👇👇👇
CLICK HERE TO GET FULL QUESTIONS AND ANSWERS
NECO 2024 PRACTICAL SPECIMEN CHEMISTRY QUESTIONS


RECOMMENDED POST:
