
Find the free and 100% verified solutions for June/July 2024 NECO chemistry OBJ and Essay questions. Keep refreshing this page for the latest updates and share with others preparing for the exam.
NECO 2024 chemistry essay and OBJ answers are now available. If you are writing this subject, here is the 2024 NECO chemistry Questions June/July verified solution. The NECO Economics Exam will commerce on 15th of July, 2024.
====================================
NECO 2024 Update
Remember
You can still subscribe for NECO.Important
The price will go up next week!Subscribe Now
Pay now and get:
Added to NECO VIP Group
Answers sent to you from midnight
Subscribe now, don’t delay!
NECO 2024 CHEMISTRY OBJ/ESSAY QUESTIONS AND ANSWERS

Papers III & II: Objective & Essay – Chemistry (3 hrs) (10:00 am – 1:00 pm)
NOTE: NECO June/July 2024 Free Chemistry OBJ & ESSAY Question and Answer Room: Pay just N1,500! Click on the link below to WhatsApp us and get the answers at 12 midnight. Prepare effectively and ensure your success in the exam with our comprehensive resources.
CLICK HERE TO GET FULL QUESTIONS AND ANSWERS
2024 NECO CHEMISTRY OBJ (OBJECTIVE) ANSWERS
================================
CHEMISTRY OBJ
01-10: BC**********E
11-20: DD**********D
21-30: AC*********AB
31-40: BDC*********B
41-50: CABA*******D
51-60: BBDDC******C
To get complete OBJ!!!
CLICK HERE TO GET FULL ANSWERS
================================
2024 NECO CHEMISTRY ESSAY (THEORY) ANSWERS
================================
CHEMISTRY ESSAY
NUMBER 1
(1a)
(i) Hydrogen gas is colorless and odorless.
(ii) Hydrogen is highly flammable, which means it can easily catch fire when exposed to a flame or spark.
(iii) Hydrogen gas has low density, making it lighter than air.
(1b)
(I) Group 17 (halogens)
(II) lodine
(III) lodine
(1ci)
I. Calcium (Ca²⁺)
II. Magnesium (Mg²⁺)
(1cii)
I. lon exchange method
II. Lime-soda method
(1di)
(I) Methyl orange is used in the titration of a strong acid with a strong base.
(II) Phenolphthalein is used in the titration of a strong base with a strong acid.
(1dii)
(I) Hydrated salt: Copper(II) sulfate pentahydrate (CuSO₄.5H₂O)
(II) Acidic salt: Ammonium chloride (NH₄Cl)
(III) Basic salt: Sodium carbonate (Na₂CO₃)
(1ei)
2-methylbutane –> pentane –> 2,2-dimethylpropane
(1eii)
(I) 1-bromo-3-methylbutane
(II) 2-methylpentane
(1eiii)
Benzene is used as a starting material in the production of various important chemicals such as ethylbenzene, cumene, cyclohexane, and nitrobenzene.
(1eiv)
Saponification is a process in which ester molecules are hydrolyzed in the presence of a strong base (such as sodium hydroxide or potassium hydroxide) to form alcohol and the salt of the carboxylic acid.
(1f)
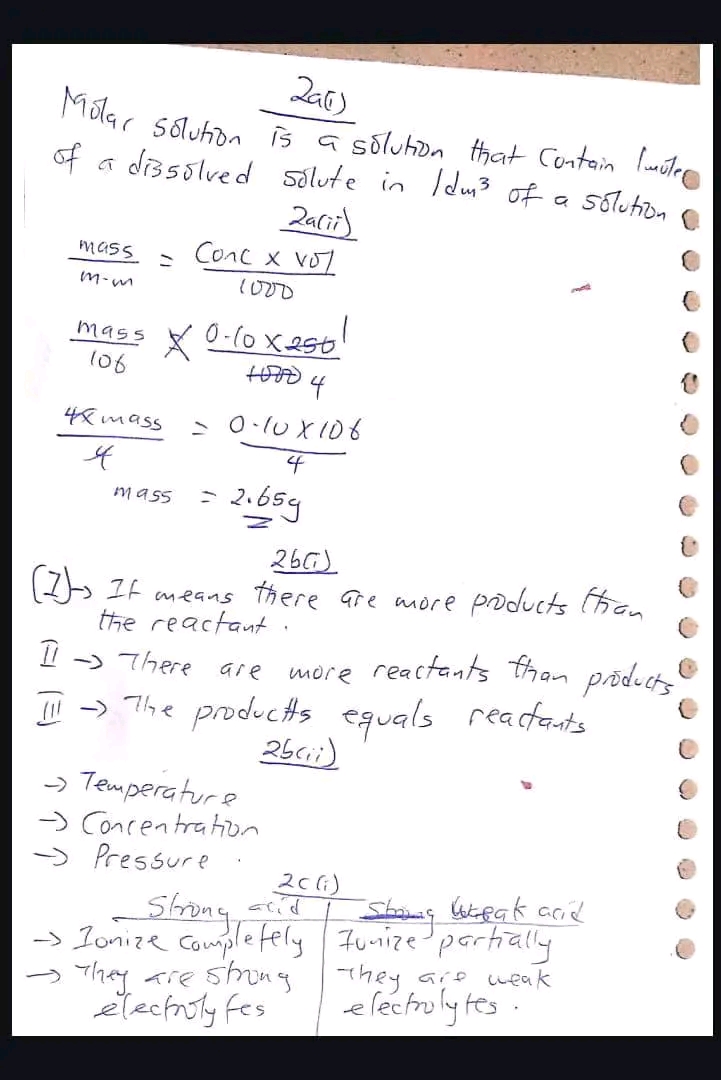
Given, Vapour density = 77
molecular mass of XCl₄ = Molecular mass = Vapour density x 2 = 77 x 2 = 154
Molecular mass of XCl₄ = X + 4(Atomic mass of Cl)
154 = X + 4(35.5)
X = 154 – 142
X = 12
:. The relative atomic mass of X is 12.
================================
Keep refreshing this page
Pay ₦1,500 And Get All Answers Now
CLICK HERE TO GET FULL ANSWERS
NECO 2024 CHEMISTRY (ESSAY/OBJ) QUESTIONS:

More CHEMISTRY QUESTIONS AVAILABLE
====================================
Recommended post:

